Digital Innovation Coming Soon to Incontinence Care
For more than 40 years, artificial sphincters have been the standard of care for stress urinary incontinence in men following prostate surgery. The iPhone age is finally catching up.
Electronic devices now in development could make it easier to control urination and enable more people to use this treatment. One-year data from a pilot study of the most advanced electronic device, UroMems’ UroActive, were presented on April 26 at American Urological Association (AUA) 2025 Annual Meeting. Researchers also presented results of a long-term outcomes trial of Boston Scientific’s manual hydraulic artificial urinary sphincter (AUC), the AMS 800, the gold standard for treatment of male stress urinary incontinence. The findings showed the evolution of incontinence treatment and the benefits and potential risks of new and existing technologies.
“Really the monumental and truly transformational aspect of this space is going to be with the innovations that will come with an electronically actuated device,” Melissa Kaufman, MD, PhD, professor of urologic surgery and chief of the Division of Reconstructive Urology and Pelvic Health at Vanderbilt University Medical Center, in Nashville, Tennessee, told Medscape Medical News. “It’s going to open up an opportunity for many more patients.”
Benefits and Risks
Stress urinary incontinence may occur after prostate surgery or radiation therapy for cancer, which can damage the nerves and muscles around the bladder. An AUS mimics the function of the normal sphincter by opening and closing the urethra under the control of the patient.
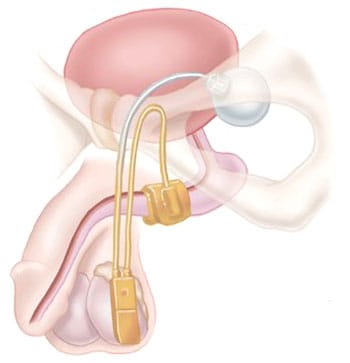
The AMS 800 system has a cuff that wraps around the urethra to control the flow of urine. A pump is implanted in the scrotum, which a patient squeezes to move fluid in the cuff to a balloon implanted in the abdomen. When the cuff is deflated, the urethra opens so the patient can pee and after a few minutes the cuff refills and closes the urethra automatically.
The prevalence of stress urinary incontinence following prostatectomy ranges from 6% to 69% depending on the study and how incontinence is defined. Ten-year follow-up data from the Comparative Effectiveness Analysis of Surgery and Radiation After Localized Prostate Cancer (CEASAR) study found up to 25% of men who underwent prostatectomy reported bothersome leakage.
Sarah Faris, MD, associate professor of surgery at the University of Chicago, Chicago, said AUS implantation is highly effective for treating severe stress urinary incontinence. After prostate surgery, men may go through two to seven heavy pads a day to absorb urine, she said; AUS implantation can get them dry or close to dry. Some people avoid behaviors and social activities because they are embarrassed about leaking and AUS “can really give people’s lives back,” Faris said.
Faris, a moderator at the AUA podium session on male incontinence, noted that the device typically lasts about 7-10 years and may need revisions. The potential risks include infection and erosion of the device into the urethra, which range between 1% and 3%, she said.
The AMS 800 device was cleared by the US Food and Drug Administration (FDA) in 1983 under the 510(k) process. In 2000, the agency issued a final rule requiring submission of a premarket approval application, which requires clinical studies, for an implanted mechanical/hydraulic urinary continence device. American Medical Systems, then the manufacturer of the device, submitted the application, which the FDA approved in 2001. Boston Scientific acquired the device from American Medical Systems in 2015.
The AUA’s guideline on incontinence after prostate treatment, which the group amended in 2024, calls for clinicians to discuss the option of AUS in patients who are experiencing mild to severe stress urinary incontinence.
Advance of Electronic Devices
While the AMS 800 has undergone modifications over the years, including changes in cuff size and the addition of antibiotic coating to minimize prosthetic infection and explant risks, its basic design has remained largely unchanged. More advanced alternatives are on the horizon.
A June 2023 article in Translational Andrology and Urology described several artificial sphincters in development, including UroMems’ device and Affluent Medical SA’s electronic Artus system. The French company announced in March 2024 it had treated the first male patient with the Artus.
The paper also noted Boston Scientific has an electronic AUS program that features personal device integration for bluetooth control. A representative for Boston Scientific declined to share details about the company’s electronic sphincter.
At the AUA meeting, Emmanuel Chartier-Kastler, MD, PhD,aprofessor of urology at Sorbonne Université in Paris, presented results of UroMems’ pilot study in six men who were 1 year out from activation of the device. All patients reported more than a 50% reduction in the combined weight for all the pads required during a 24-hour period (the 24-hour pad weight test).
Chartier-Kastler, the study’s principal investigator, said in an interview he has used the mechanical hydraulic AUS for 30 years. Some of his patients complain the device is too complicated, and many do not want to manipulate a pump. The main advantage of UroActive is patients “just have to use a patient remote control like you use for your garage door,” he said.
The wireless remote-control unit has two settings, a “baseline” or ambulatory setting and a “lying down” setting that puts less pressure on the occlusive cuff. The settings are fixed in consultation with the patient’s physician so the patient can switch from one setting to another. Chartier-Kastler said the device may result in fewer urethral complications because it generates less pressure on the urethra when the patient is lying down. In addition, he said the system will automatically open the urethra so it does not depend on the patient, who may have cognitive or physical impairments, to do so.
UroMems said it will begin enrolling approximately 100 men in a pivotal trial at multiple sites in France and the United States later this year. The results of the study will be the basis of a premarket approval application to FDA, according to the company, which plans to seek an indication for men with urinary incontinence due to intrinsic sphincter deficiency, including those following prostate surgery. UroMems said it also intends to conduct a pivotal trial of the device in women with urinary incontinence.
In another presentation on patient interest and concerns about electronic urinary sphincters, Yannic Volz, MD, an assistant professor at Ludwig-Maximillans-Universität in Munich, Germany, described the results of a survey of 208 men who underwent implantation of an AUS. Among them, 166 (79.8%) said they were satisfied with their current device, 186 (89.4%) would opt for the procedure again, and 125 (60.4%) expressed interest in an electronic system.
Most patients in the survey said they preferred remote control operation of the electronic AUS, although some reported being concerned about potential malfunctions, such as battery failure or loss of connection between the device and its controller. An article about the survey published in European Urology Open Science in February reported that 60% of respondents said they preferred smartphone-based control.
Prospective Clinical Outcomes Study
Although the medical literature contains many studies of AUS outcomes, most of these have been single-center, retrospective analyses of the technology. A group of urologists recently conducted a multi-institutional prospective AUS clinical outcomes (AUSCO) trial to assess safety and efficacy of the AMS 800 device, and quality of life in patients who receive it. The results of the single arm study of 115 men at 17 sites in the United States and Australia, were presented at the AUA meeting but have not yet been published.
Kaufman, the national principal investigator of the trial, reported the study met the primary endpoint, with 93.8% of patients experiencing at least 50% reduction in the weight of pads used in 24-hour period at 12 months. A total of 60.4% of men reported using no pads at the 12-month mark.
Adverse events were fairly common, with 38 patients (33%) reporting at least one complication during the study: 10 (8.7%) experienced serious problems with the device; nine (7.8%) required revision implantation; two (1.7%) experienced erosion; and three (2.6%) experienced a mechanical malfunction. No infections related to the AUS were reported.
Kaufman said previous retrospective studies were limited by variability in the way researchers measured outcomes.
“The regulatory landscape in the country has changed since the introduction of the artificial sphincter, and now we need to have very robust datasets that are standardized in order to move forward with any future technologies,” she said.
Kaufman noted that, in addition to the significant reduction in the need for pads and the 0% infection rate, men with a history of radiation therapy to the pelvis, who accounted for almost a quarter of participants in the trial, were not at increased risk for poor outcomes or adverse events.
Based on retrospective studies in the literature one “would expect that irradiated patients may have higher levels of erosion, explant and secondary surgeries,” she said. “We’ll have to follow these men out for a longer period of time to assess impact, however for the time frame of this study, radiation did not affect outcomes or increase adverse events, which was very reassuring.”
In other analyses of the AUSCO data, Andrew Peterson, MD, a professor of surgery at Duke University in Durham, North Carolina, and a co-investigator on the trial, described patient perceptions about ease of use after AUS implantation and the device’s improvement in depression, anxiety, and overall emotional health in men with stress urinary incontinence.
AUS in Women
Artificial sphincters have not been widely used for treatment of stress urinary incontinence in women. One reason, Faris said, is slings, surgical mesh implanted to support the urethra or bladder neck, are so effective an AUS typically is unnecessary.
Kaufman noted implantation in women is more challenging as the cuff is implanted at the bladder neck rather than the urethra, which traditionally requires an open incision. And the pump, which is placed in the labia, can sometimes be uncomfortable to manipulate.
Still, research on the devices in women is ongoing. Researchers at University Hospitals Coventry and Warwickshire NHS Trust in the United Kingdom, presented a systematic review and meta-analysis of clinical outcomes and safety of AUS for women with stress urinary incontinence. The analysis of eight studies comprising 300 patients found implantation of the devices was associated with an overall rate of continence of 72%. Rates of revision (22.5%), explant (17.6%), and overall postoperative complications (26.3%) were moderate, the UK researchers reported.
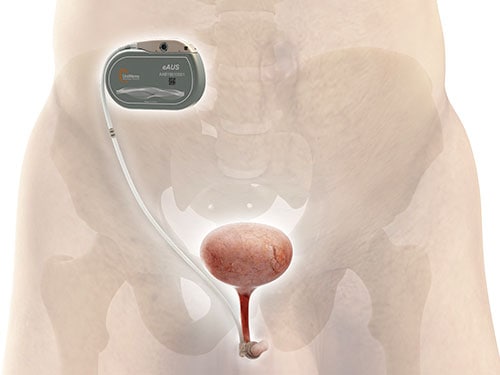
UroMems announced results of its first clinical feasibility study of UroActive in women in February. The study was conducted in France in six women with stress urinary incontinence. The company said at 6 months after implantation the devices operated as expected with no need for revision or explant.
Kaufman said robotic surgery is now being used to implant the device in women in a much less invasive way. And with the emergence of an electronic device, she said, “you won’t have to have the labial pump which should open access for a lot of women.”
Chartier-Kaster is a paid consultant for UroMems. Kaufman was the national principal investigator for the AUSCO trial, which was funded by Boston Scientific, and received compensation from Boston Scientific for serving as faculty on its courses. Other sources reported no relevant financial conflicts of interest.
Brenda Sandburg is a freelance journalist who has written about US Food and Drug Administration policies, the biopharmaceutical industry, and legal issues for the Pink Sheet and American Lawyer Media.


 Admin_Adham
Admin_Adham


